If you have any questions about our products, please question submitted to us, we will be the first time to answer your questions!
What is the difference between molecular sieve and zeolite?
A molecular sieve is a material containing tiny pores of a precise (0.3-2 nm) and uniform size that is used as an adsorbent for gases and liquids. Molecules small enough to pass through are adsorbed, while larger molecules are not. It is different from a common filter in that it operates on a molecular level. Any materials that have relative uniform pore size of molecular level can be regard as "molecular sieve". Examples include zeolites molecular sieve, carbon molecular sieve. Most molecular sieves in practice today are zeolites.
Zeolites are microporous, aluminosilicate. The term zeolite was originally invented in 1756 by Swedish mineralogist Axel Fredrik Cronstedt, who observed that rapidly heated material produced large amounts of steam from water that had been adsorbed by the material. Based on this, he called the material zeolite, from the Greek, meaning "to boil" and , meaning "stone". Zeolites occur naturally but are also produced industrially on a large scale. (Wikipedia)
Zeolites are crystalline aluminosilicates of group IA and group IIA elements, such as sodium, potassium, magnesium and calcium. Chemically, they are represented by the empirical formula:
M2/n O.Al2O3.γSiO2. ώH2O
Where y is 2 – 200, n is the cation valence and w represents the water contained in the voids of the zeolite. Structurally, zeolites are complex, crystalline inorganic polymers based on an infi nitely extending three - dimensional, four - connected framework of AlO 4 and SiO 4 tetrahedra linked to each other by the sharing of oxygen ions. As of September 1980, 232 unique zeolite frameworks have been identified, and over 40 naturally occurring zeolite frameworks are known. Every new zeolite structure that is obtained has to be approved by the International Zeolite Association Structure Commission and receives a three letter designation.
What is a desiccant air dryer?
Desiccant air dryers are typically designed to work efficiently in critical applications were dew points of -40°F to -100°F are needed.Desiccant air dryers; use desiccant beads such as activated alumina, silica gel, or molecular sieves 4A to adsorb the moisture found in the compressed air stream. Desiccant air dryers are engineered to remove water vapor in compressed air systems to dew points as low as -100°F. Desiccant air dryers are typically found in industries such as; food, pharmaceuticals, painting, paper and pretrochemicals that have very specific drying requirements.

Mingguang Feizhou. is a professional manufacturer of Molecular Sieves, activated alumina since 1980. Any questions about the desiccants, please feel free to contact us.
What molecules does molecular sieve adsorbed and excluded?
Zeolite adsorbents are synthetically produced molecular sieves that are microporous, crystalline, metal aluminosilicates. The uniform crystalline structure of molecular sieve adsorbents provides very predictable and reliable adsorptive properties. Metal cations contained in the crystalline structure of molecular sieve adsorbents balance the negative charge of the framework. These metal cations create an electrical field, hence their strong affinity for polar molecules.

Depending on the type of crystalline structure and the occupying cation of the molecular sieve adsorbent,molecules may be readily adsorbed or completely excluded according to their relative molecular size. For example, a 3A molecular sieve is particularly useful for the dehydration of olefins. It will adsorb water, but will exclude an olefin molecule. Zeolite molecular sieve adsorbents have a strong affinity for polar and polarizable molecules and even exhibit a selective preference for various polar molecules. Based on their degree of polarity. Water is the most polar molecule known, and is therefore the most preferred and strongly adsorbed onto zeolite molecular sieves.
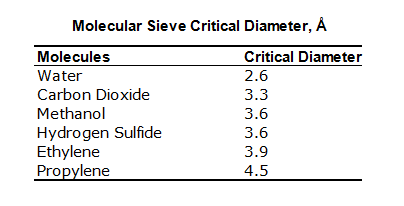
The following is a table list of molecules different types of molecular sieve adsorb and exclude:
Molecule Sieves: Molecules Adsorbed and Excluded
| Type | Molecules Adsorbed** | Molecules Excluded |
| 3A | Molecules with an effective diameter <3 angstroms including H2O and NH3 | Molecules with an effective diameter >3 angstroms (ethane) |
| 4A | Molecules with an effective diameter <4 angstroms including ethanol, H2S, CO2, SO2, C2H4, C2H6 and C3H6 | Molecules with an effective diameter >4 angstroms (propane) |
| 5A | Molecules with an effectivediameter <5 angstroms including nC4H9OH, n-C4H10, C3H8 to C22H46 and R-12 | Molecules with an effective diameter >5 angstroms (propane) (iso compounds and all 4-carbon rings) |
| 13X | Molecules with an effective diameter <8 angstroms including benzene and toluene | Molecules with an effective diameter >8 angstroms (C4F9)3N |
Chart depicts basic molecular sieve types only. In all applications, these basic forms are customized for specific use.
Each type adsorbs listed molecules plus those of preceding type.
Where are molecular sieve used in natural gas liquification?

In an LNG plant, natural gas from the wellhead must be treated before liquefaction can take place. This treatment happens in dedicated units where contaminants like water, mercury and sour components are removed. These treating facilities are an essential part of LNG plants, helping ensure reliable LNG production.
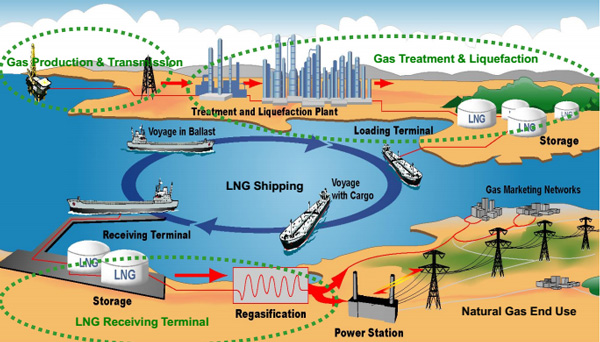
In a typical LNG plant, a split is made between warm and cold units, as these units can be independently located and operated. The cold units are typically operated at cryogenic conditions. In the treating facilities, front-end processing of the raw natural gas occurs to meet the strict feed gas specifications for liquefaction. The order of connection between the different units is determined by the feed gas requirements and the technology selected for each unit.
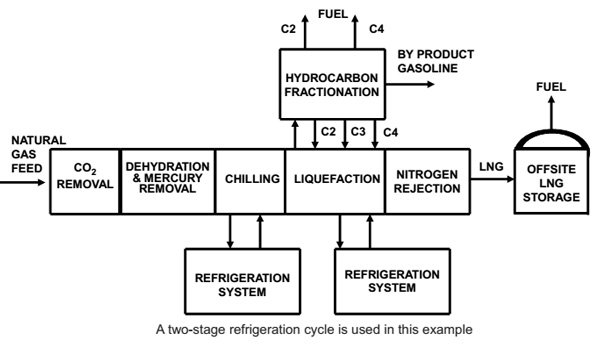
The feed gas from upstream typically arrives in a slug catcher before the gas is routed, via a knockout vessel, to the first gas treating unit, the acid gas removal unit (AGRU). To ensure problem-free operation of the AGRU, it is essential to have proper liquid knockout and to prevent carryover of liquid hydrocarbons to the AGRU.3 In the AGRU, the CO2 content is reduced to the LNG specification, usually by an amine-based solvent. The CO2 is removed from the gas by contacting with the circulating solvent in a high-pressure absorber. The solvent is then regenerated by stripping out the CO2, using steam at low pressure.
After contacting with the solvent, the treated natural gas is water-saturated. Before liquefaction of the gas can take place, the water content must be reduced below 0.1 ppmv to prevent freeze out in the cryogenic process units.
Deep removal of water is done by molecular sieve adsorbents in the dehydration unit (DHU). Compared with other adsorbents and water-removal technologies, molecular sieves have a high water capacity at low partial water pressures, which ensures that the tight water specification can be met.
Generally, LNG plant designs require that the DHU inventory be changed out once every four years. The changeout of the molecular sieve is usually aligned with other plant maintenance, such that a dedicated shutdown is not required and the molecular sieve changeout does not determine plant availability. Mercury-free feed gas is required to prevent mercury-induced corrosion of the main cryogenic heat exchanger (MCHE), which is made of aluminum. Mercury is removed by a non-regenerable adsorbent.
NEWSLETTER SIGNUP
By subscribing to our mailing list you will always be update with the latest news from us.
We never spam!
Copyright © Mingguang Feizhou new materials Co., Ltd. All Rights Reserved. | Sitemap | Technical Support: 