If you have any questions about our products, please question submitted to us, we will be the first time to answer your questions!
How about Molecular Sieve Application in Gas Dehydration and Purification?
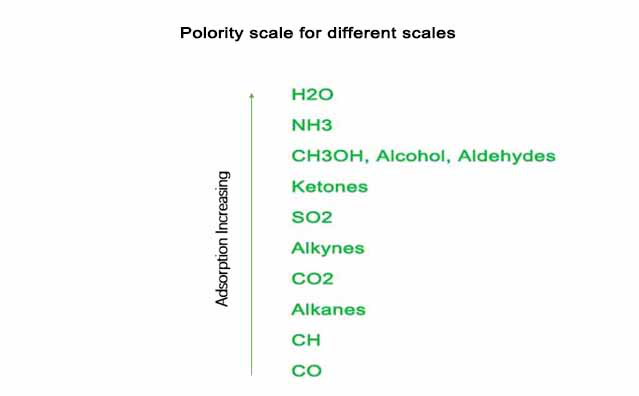
Zeolite molecular sieve is a aluminosiliate with uniform pores. These pore diameters are similar in size to small molecules. Thus molecules with diameter less than the pore size can enter molecular sieve and be adsorbed, while molecules with diameter bigger than the pore size can not go into molecular sieve and be excluded (Tab. 1). Moreover, molecular sieve pore size can be precisely adjusted through introducing of different metal ions such as Na+(4A, 13X), K+(3A), Ca+(5A, CaX), Li+(LiLSX, LiLX). This property enables molecular sieve to separate molecules of different size. Another important property of molecular sieve is it is easier to adsorb polar molecules other than non polar molecules, thus makes it possible to separate molecules by its polarity (Fig. 1).
Tab.1 pore size of different kinds of molecules
| Molecules | Critical Diameter in Å |
| Helium | 2 |
| Hydrogen, Acetylene | 2.4 |
| Water, O2, Monoxide and Dioxide | 2.8 |
| Nitrogen | 3 |
| Ammonia, Hydrogen Sulphide | 3.6 |
| Argon | 3.8 |
| Methane | 4 |
| Ethylene, Ethylene monoxide | 4.2 |
| Ethane, Methanol, Ethanol | 4.4 |
| Methyl-mercaptan | 4.5 |
| Propane, nC4 to nC22 | 4.9 |
| Propynene | 5 |
| Ethyl-RH, Butene 1, Butene 2 trans | 5.1 |
| Difluochloromethane (R22) | 5.3 |
| ISO C22 | 5.6 |
| Cyclohexane | 6.1 |
| Toluene, Paraxylene | 6.7 |
| Benzene | 6.8 |
| Carbon tetrachloride | 6.9 |
| Metaxylene | 7.1 |
| Tri-ethylamine | 8.4 |
Molecular Sieve Application
| Type | Pore Size | Application |
| 3A | 3Å | 1. Drying of unsaturated hydrocarbons 2. Cracked gas drying 3.Drying of natural gas, if COS minimization is essential, or a minimum co-adsorption of hydrocarbons is required 4. Drying of highly polar compounds, such as methanol and ethanol |
| 4A | 4Å | 1. Drying and removing of CO2 from natural gas, LPG, air, inert and atmospheric gases, etc 2. Removal of hydrocarbons, ammonia and methanol from gas streams (ammonia syn gas treating) 3. Dehydration of refrigerant and air in the air break units of buses, trucks and locomotives 4. Packed in small bags for packing desiccant for foods, etc |
| 5A | 5Å | 1. The strong ionic forces of the divalent calcium ion makes it an excellent adsorbent to remove water, CO2, H2S from sour natural gas streams, while decreasing COS formation. It can also adsorbs light mercaptans. 2. It is also used for the separation of normal- and iso-paraffins 3. Production of high purity N2, O2, H2 and inert gases from mixed gas streams 4. PSA hydrogen purification |
| 13X | 10Å | 1. Removal of CO2 and moisture from air (air pre-purification) and other gases 2. Separation of enriched oxygen from air 3. Removal of mercaptans and hydrpogen sulphide from hydrocarbon liquid streams such as LPG, butane, propane etc 4. Catalyst protection, removal of oxygenates from hydrocarbons (olefin streams) 5. Removal of n-chained compositions from aromatics |
| 13X-1 | 10Å | 1. with smaller beads is degined for small medical oxygen concentrator 2. 13X-1 is desinged for bigger industrial or medical oxygen concentrators smaller than 200 Nm3/h |
| 13X-2 | 10Å | 1. 13X-2 with smaller beads is degined for small medical oxygen concentrator; 2. JLOX-102, JLOX-103 is desinged for bigger industrial or medical oxygen concentrators larger than 200 Nm3/h |
What are zeolite molecular sieves?
Molecular sieve usually means zeolite molecular sieve, it is crystalline, highly porous materials, which belongs to the class of aluminosilicates.
The crystals of molecular sieve are characterized by a three-dimensional pore system, with pores of precisely defined diameter. This diameter is in the dimension of the size of molecules such as water, CO2 and H2S. The pores can be adjusted to precisely determined uniform openings allowing for molecules smaller than its pore diameter to be adsorbed while excluding larger molecules, hence the name "molecular sieve".
Molecular sieve has a special selective adsorption and ion exchange properties, and widely used to dehydration of gas and solvent in insulating glass, automobile air-actuated braking system, refrigeration equipment, industrial gas making, painting & coating, petrochemical industry, etc.
A 4 X 8 mesh bead is normally used in gasphase applications, while the 8X12 bead is common in liquidphase applications.
What is the difference of zeolites and molecular sieves?
Zeolites are a group of alumino-silicate minerals, with a solid crystalline structure, three-dimensional, microporous, and well-defined structures that contain aluminum, silicon, and oxygen in their regular framework. Zeolites may occur either in nature or are manufactured synthetically, most zeolites used commercially are produced synthetically. Zeolite crystals are porous, the pores have the unique characteristic of being all of the same size. The diameter of these pores can be as small as a very tiny molecule such as water.
Molecular sieves are a certain kind of zeolites, which for their pore size and chemical composition have particularly developed adsorption properties, which means to separate or remove one substance from another on a molecular scale. While the properties of natural zeolites are fixed, synthetic zeolites can be tailored to meet industrial specifications. Therefore, synthetic zeolites dominate the molecular sieve market among which the "A" and "X" structures are the most commonly used.
How to Regenerate Molecular Sieve?
Regeneration Molecular Sieve should be in typical cyclic systems constitutes removal of the adsorbate from the molecular sieve bed by heating and purging with a carrier gas.
Sufficient heat must be applied to raise the temperature of the adsorbate, the adsorbent and the vessel to vaporize the liquid and offset the heat of wetting the molecular-sieve surface. The bed temperature is critical in regeneration. Bed temperatures in the 175-260℃ range are usually employed for type 3A. This lower range minimizes polymerization of olefins on the molecular sieve surfaces when such materials are present in the gas. Slow heat up is recommended since most olefinic materials will be removed at minimum temperatures, 4A, 5A and 13X sieves require temperatures in the 200-315℃ range.
After regeneration, a cooling period is necessary to reduce the molecular sieve temperature to within 15℃ of the temperature of the stream to be processed.
For optimum regeneration, gas flow should be countercurrent to adsorption during the heat up cycle and concurrent (relative to the process stream) during cooling.
Alternatively, small quantities of molecular sieves may be dried in the absence of a purge gas by oven heating followed by slow cooling in a closed system, such as a desiccator.
How to produce attapulgite binder and molecular sieve in Mingguang Feizhou
Molecular sieves has uniform inner pores, the pores' diameter is the same with some molecules. Our products includes molecular sieves and synthetic zeolite powders with type 3A, 4A, 5A, 10X, 13X and etc. And each type can be detailed to many kinds. We will introduce them separately.
Because synthetic zeolite powder 3A and 5A was transformed from synthetic zeolite powder 4A by ion exchange, and synthetic zeolite powder 10X was transformed from synthetic zeolite powder 13X by ion exchange, and also the processes of making synthetic zeolite powder 4A and 13X are the same, so we will start from the manufacturing process of synthetic zeolite powder 4A.
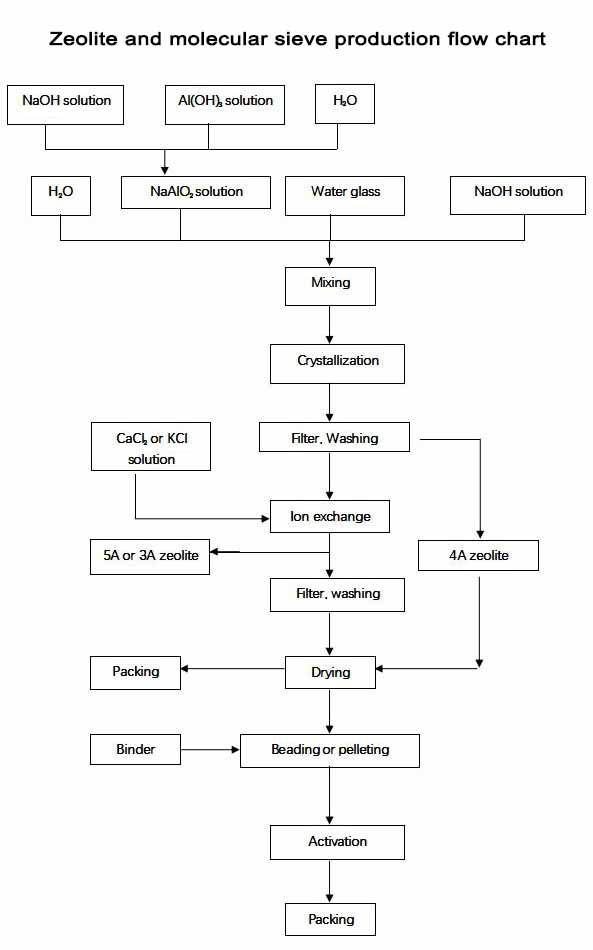
The manufacturing process of synthetic zeolite powder 4A can be divided into material preparing, mixing, crystallization, filter, washing, drying and packing. The making method is called hydrothermal synthesis method.
And the manufacturing process of molecular sieves is as below, adding binders to the synthetice zolite powders, after beading or pelleting, calcination and packing, the products came to be OK.
The raw materials for manufacturing synthetic zeolite powders are the same including NaOH, Al(OH)3, water and water glass (Na2SiO3 solution), we have several tanks in the manufacturing department which each tank has one big letter on the body, A, H, S, H, they mean NaAlO2 solution, NaOH solution, (Na2SiO3 solution) and water seperately, and the binders for molecular sieves' beading or pelleting are Kaolin Clay and Attapulgite Clay. The process is as the belowing graph.
The material of solid Al(OH)3 and NaOH we used in manufacturing are high quality, it can be used only when these solid materials are making into solution by adding some water. After mixing the Al(OH)3 solution and NaOH solution by certain rate, we got NaAlO2 solution, it is stored in the tank named A. We buy Na2SiO3 solution from another big company, it is also high quality and stored in the tank named S in the manufacturing department.
Then we mixing NaAlO2 solution, NaOH solution, Na2SiO3 solution and water by certain rate in the mixing tank, after we heat the tank for a certain time, we got the raw 4A crystal, and then we put the 4A crystal into the filter tank for filter and washing, and also for drying, after these processes, we got the synthetic zeolite powder 4A, and after packing in the packing department, we got the final 4A synthetic zeolite powder.
For the producing process for synthetic zeolite powder 5A and 3A, it is much the same with 4A synthetic zeolite powder, after we got the 4A crystal, we put the 4A crystal into the ion exchange tank, if we want get 5A, we put the CaCl2 solution in, if we want 3A, we will put the KCl solution in, at the same time, we heat the ion exchange tank. After filter, washing, drying and packing, we got the final 5A or 3A synthetic zeolite powder.
And the manufacturing process of 13X synthetic zeolite powder is the same with 4A synthetic zeolite powder, only the mixing rate is different. And after we got 13X crystal, we put the srystals into the ion exchange tank, and also put the CaCl2 solution in, after heating, filter, washing, drying and packing, we got the final synthetic zeolite powder 10X.
The manufacturing of molecular sieve is much easier, we add some binders to the synthetic zeolite powders with certain rate, usually we use Attapulgite clay and Kaolin clay as the binders. And also we will add some water. If we need bead products, we will put the well mixed synthetic zeolite and binders to the rolling machine, and the powders will be formed to bead, the diameter of the bead will be well controlled arrcording to the customers' need, this is called products' beading, after the beads are pre-dryed in the air, we will put the beads to the calcination oven, after calcination, we got the hot products, only after beads are cooling for a short time and packing, we got the final bead products; if we need pellet products, we will put the well mixed synthetic zeolite and binders to the pelleting machine, arrording to customers' needs, we will choose the 1/8''1 or /16'' pellet, after pelleting, we put the pellets into the calcination oven, in the exit of the oven, we got the final pelleting products, after packing, it can be sold.
And we can see the products manufacturing graph as below, it will be much clearer:
How to get anhydrous and fuel ethanol?
Through distillation alone, ethanol can only to dehydrated to around 95% purity, with the remaining water unable to be removed due to the formation of an azeotrope, leaving the ethanol unsuitable as fuel.
Dehydration with azeotropic distillation
Most of the ethanol dehydration plants for production of anhydrous ethanol are based on Azeotropic distillation. It is a mature and reliable technology capable of producing a very dry product. However, its high capital cost, energy consumption, reliance on toxic chemicals like benzene and sensitivity to feedstock impurities, has virtually eliminated the use of azeotropic distillation in modern ethanol plants. Benzene has been used as entrainer of choice of ethanol dehydration but it is now known to be a powerful carcinogen.
Dehydration with Molecular Sieve Process
Ethanol can be applied in PSA or VSA dehydration units and is used to remove water in ethanol streams after distillation. This molecular sieve has been specifically designed for Ethanol Dehydration Units and differs from standard molecular sieves. With a high crush strength to withstand the heat and pressure of the dehydration unit, the design of Ethanol dehydration beads maintains a low bulk density, while offering a high working capacity.3A Zeolite, has a superior selectivity between water and ethanol molecules, which increases both efficiency and dehydration capacity each cycle. Once ethanol has been dehydrated, it is about 99.99% pure and can then be applied in biofuels for direct blending and ETBE production, or for other uses such as in chemicals, food industry, pharmaceuticals, and more industries.
NEWSLETTER SIGNUP
By subscribing to our mailing list you will always be update with the latest news from us.
We never spam!
Copyright © Mingguang Feizhou new materials Co., Ltd. All Rights Reserved. | Sitemap | Technical Support: 